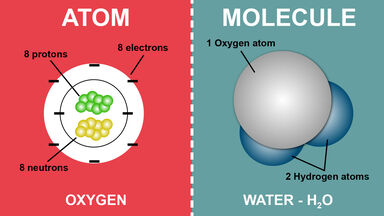
1.
Insulin contains 3.4% sulphur. The minimum molecular weight of insulin is –
2.
The hydrogen phosphate of a metal has the formula MHPO4. The formula of its chloride would be
3.
A piece of sodium weighs 0.023 g. The number of atoms present in it are
4.
The largest number of molecules among the following is –
5.
Read the Question given below
6.
The hemoglobin from the red blood corpuscles of most mammals contains approximately 0.33% of iron by weight. The molecular weight of hemoglobin is 67,200. The number of iron atoms in each molecule of hemoglobin is (Atomic weight of iron = 56):
7.
X and Y atoms have 2 and 6 valence electrons in their outermost shells respectively. The compound which X and Y are likely to form is –
8.
Ritish needs 1.71 g of sugar (C12H22O11) to sweeten his tea. What would be the number of carbon atoms present in his tea?
9.
A compound having the empirical formula (C3H4O) has a molecular weight of 168. The molecular formula of this compound is –
10.
Number of molecules present in 0.18 g H2O are