DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
POINTS TO REMEMBER
Bonding In Carbon – The Covalent Bond
■ Covalent bond is formed by mutual sharing of electrons between the two atoms so that each of the atoms attains noble gas configuration.
■ Covalent bond can be single, double or triple bond depending upon the number of shared pairs of electrons between them.
■ Carbon shares its valence electrons with atoms of various elements to form a variety of compounds.
■ Carbon can form single, double and triple covalent bonds with itself as well as with other atoms.
■ Covalent compounds generally have low melting and boiling points, low solubility in water and are non-conductors of electricity.
Table of Contents
QUESTIONS ON CARBON AND ITS COMPOUNDS
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
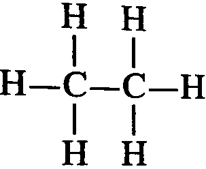
1. How many covalent bonds are present in a molecule of ethane?
Ans. The bond structure of ethane molecule is
2. Carbon forms only covalent bonds. Give reasons.
Ans. Carbon (Z = 6) has electron distribution 2, 4. In order to acquire octet of electrons it can neither gain 4 electrons nor lose 4 electrons because of high amount of energy required for such processes. Hence it shares its valence electrons with other atoms to form covalent bonds only.
3. Draw the electron dot structures of following molecules.
Ans. (i) Ethanoic acid
(ii) H2S
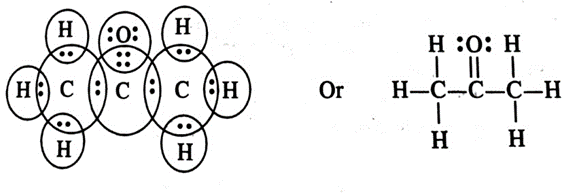
(iii) Propanone
(iv) Chlorine
Ans. (i) Ethanoic (CH3COOH)
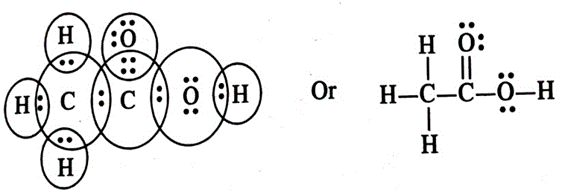
(ii) H2S
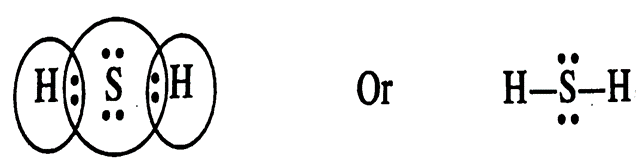
(iii) Propanone (CH3COCH3)
(iv) Chlorine (Cl2)
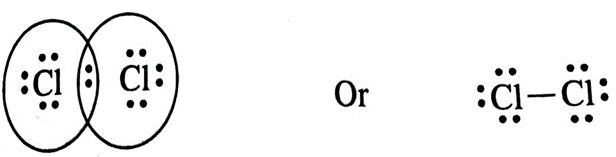
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
4. Explain giving reason why carbon neither forms C4+ cations nor C4- anions, but forms covalent bonds which are bad conductors of electricity and have low melting point and low boiling point.
Ans. Carbon (Z = 6) have four electrons in its valence shell. it can attain noble gas configurationeither by gaining four electrons to form C4- ion or lose four electrons to form C4+ ion. In boththese processes a very high amount of energy is required. Hence formation of C4+ or C4-ionsis not possible. Therefore carbon atom attains octet of electrons by sharing its four valenceelectrons with other atoms to form covalent compounds
The covalent compounds cannot conduct electricity and also have low melting and boiling points due to weak intermolecular forces.
5. Why are most carbon compounds poor conductors of electricity?
Ans. Carbon compounds are generally covalent compounds because carbon atom shares its four valence electrons to form covalent bonds with other atoms. Carbon compounds therefore do not contain ions. They do not ionise and hence, they are not good conductors of electricity
Versatile Nature of Carbon
■ Carbon is most significant and versatile element which forms the basis for all living organism and many of the things that we use or consume to sustain our lives.
■ Carbon always exhibit a covalence of four in all its compounds.
■ Carbon has unique self linking property called catenation due to which it can form long chain as well as branched chain compounds.
■ Carbon exist in three crystalline allotropic forms, namely; diamond, graphite and fullerenes. Compounds of carbon and hydrogen are called Hydrocarbons which can be further categorized into saturated and unsaturated hydrocarbons.
■ Structurally similar organic compounds are grouped together to constitute a family called homologous series.
■ Different families and their structures and names are derived from the saturated hydrocarbons called alkanes.
■ Atom or group of atoms which determine most of the properties of organic compound is called functional group.
■ The compounds having same molecular formula but different structural arrangement are called isomers.
6. Write the formula of next homologue of CH3COCH3
Ans. The next homologue of CH3COCH3 is CH3COC2H5 Called butanone.
7. Write the structural formula of hexanal.
Ans. The structural formula of hexanal is:
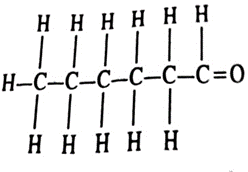
CH3CH2CH2CH2CH2CHO
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
8. Draw the structural formula of butanone molecule.
Ans. The structural formula of butanone is
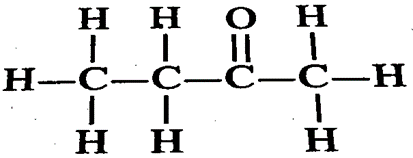
9. Write the next homologues of propanol (CH3CH2CH2OH) and butanal (CH3CH2CH2CHO)
Ans. Next homologue of
Propanol is: CH3CH2CH2OH (butan-1-ol) and that of
Butanal is: CH3CH2CH2CHO (pentanal)
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
10. Why are homologous series of carbon compounds so called? Write chemical formula of two consecutive members of a homologous series and state the part of these compounds that determines their
(i) Physical properties and
(ii) Chemical properties
Ans. Homologous series are so called because they contain structurally similar carbon compounds which can be represented by same general formula. They also have same methods of preparation and similar chemical properties. Any two consecutive members of homologous series have common difference of -CH₂. For example, two consecutive members of alcohols are C₂H5OH (ethanol) and C3H7OH (Propanol).
(i) The physical properties are generally determined by alkyl part.
(ii) The chemical properties are generally determined by the functional group.
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
11. What are the two properties of carbon which lead to huge number of carbon compounds that we see around us?
Ans. 1. Catenation It is the self linking property of carbon atom due to which it can form straight chains, branched chains as well as ring structures of different sizes.
2. Tetra covalency It always exhibits a valency of four and also has the ability to form single, double as well as triple bonds.
12. Catenation is the ability of an atom to form bond with other atoms of same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of two elements. Give reason.
Ans. Both carbon and silicon have similar configurations of valence shell i.e., each one has four valence electrons. Hence both exhibit-tendency of catenation. However, carbon being smaller in size forms relatively stronger C-C bond than Si-Si bond. As a result, carbon shows catenation tendency to a greater extent.
13 (a) Define homologous series of carbon compounds.
(b) Why cannot we have isomers of first three member of alkane’s family?
Ans. (a) Homologous series is a series of structurally similar organic compounds containing same functional group and having same general formula. Each member of the series differs from the previous and the succeeding member by a common difference of CH₂.
(b) There is no scope of branching in first three members of alkane series, namely; methane (CH4) ethane (C2H6) and propane (C3H8). Hence no structural isomerism is possible
14. Write the higher homologue of the following
(i) C3H8
(ii) C4 H8
Ans. (i) C4 H10
(ii) C6 H10
Chemical Properties of Carbon Compounds
■ Carbon and its compounds are major sources of fuels because they burn in air to produce CO2 and water along with lot of heat energy.
■ The burning of carbon compounds in air is called combustion reaction which is always exothermic.
■ Saturated hydrocarbons generally give substitution reactions which involve the replacement of an atom or group of atoms by other atoms or functional groups.
■ Unsaturated hydrocarbons such as alkene and alkynes generally give addition reactions which involved addition of simple molecules such as H2, Cl2, HCl, etc., across the double (C = C) or triple bond (C =C).
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
15. Name the products formed by complete combustion of ethanol
Ans. Carbon dioxide and water.
16. Unsaturated hydrocarbons show addition reactions but saturated hydrocarbons don’t why?
Ans. Unsaturated hydrocarbons contain one or more (double bonds) C = c or (triple bonds) C = C in their molecules. These multiple bonds become the cause of reactive nature of unsaturated hydrocarbons, they give addition reactions readily.
H2C = CH2 + H2 CH3 CH3
(H2 adds across C = C bond)
Saturated hydrocarbons contain only C C or C H bonds, hence, they cannot undergo addition reactions.
17 How do melting and boiling points of the hydrocarbons change with increase in molecular mass?
Ans. The increase in the molecular mass of the hydrocarbons results in the increase in their molecular size which increases the magnitude of intermolecular forces of attraction. Consequently, the melting and boiling points of the hydrocarbons also increase.
18. Explain the given reactions with the example
(a) Hydrogenation
(b) Oxidation
(c) Substitution
(d) Saponification
(e) Combustion
Ans. (a) Hydrogenation: This involves addition of H2 molecule across C = C or C = C bonds in unsaturated molecules. For example,
HC CH + 2H2 CH3 CH3 473k
(b) Oxidation: This involves oxidation of organic molecule by suitable oxidizing agent. For example
CH3CH2OH CH3 CH3 H2 S04
(c) Substitution: It involves replacement of an atom or group of atom from organic molecule by other groups. For example,
CH3 CH3 + Cl2 CH3CH2Cl + HCl Ethane Chloromethane
(d) Saponification: It involves alkaline hydrolysis of esters.
CH3COOC2H5 + NaOH CH3COONa + C2H5OH
Ethyl acetate Sodium ethanoate Ethanol
(e) Combustion: It involves burning of organic compound in oxygen
2C2H6 (g) + 7O2 (g) 4CO2 (g) + 6H2O
19. Give one example with chemical equation for the following types of reactions.
(a) Substitution reactions
(b) Saponification reactions
(c) Combustion reactions
(a) Substitution reactions: Such reactions involve replacement of an atom or group of atoms form an organic molecule with another similar atom or group.
CH4 (g) + Cl2 (g) CH3Cl + HCl Methane (Methyl chloride)
(Here, H atom is substituted by Cl atom)
(b) Saponification reactions: It is alkaline hydrolysis of ester molecules.
CH3COOC2H5 (l) + NaOH (aq) CH3COONa (aq) + C2H5OH (l)
Ethyl acetate Sodium ethanoate Ethanol
(c) Combustion reactions: These reactions involve burning of organic substance in oxygen to form CO2 and water.
2C2H2 (g) + 5O2 (g) 4CO2 (g) + 2H2O (l)
Ethyne
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
20. Why do alkanes burn with blue flame?
Ans. Alkanes are saturated hydrocarbons. During burning they undergo complete combustion and no unburnt carbon particles are emitted. Hence they burn with clean blue flame.
21. A mixture of oxygen and Ethyne is used for welding. Can you tell why a mixture of Ethyne and air is not used?
Ans. Ethyne is an unsaturated hydrocarbon. Its combustion in air, (i.e., lesser amount of oxygen) produces yellowish flame with lot of black smoke due to unburnt carbon particles. In other words, the combustion is incomplete and heat produced is less which does not help to attain the high temperature for temperature. Hence, to ensure complete combustion a mixture of Ethyne and oxygen is used.
22. Why pentane has higher boiling point than methane?
Ans. Molecular size of methane (Mol. mass = 16 u) is smaller than that of pentane (Mol. mass =72 u). Thus, intermolecular attractive forces in pentane are relatively stronger as compared to methane. Hence, boiling point of pentane is higher.
Some Important Carbon Compounds-Ethanol and Ethanoic Acid
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
■Ethanol is C₂H5OH and is also commonly called ethyl alcohol.
■Alcohol containing 95% ethanol and 5% water is called rectified spirit while 100% pure ethanol is called absolute alcohol.
■Ethanol can react with sodium metal to liberate H₂ gas. It also produces ethane by reacting with concentrated sulphuric acid at 443K.
■Oxidation of ethanol with potassium permanganate gives another important organic compound acetic acid (CH3COOH) also called Ethanoic acid.
■Ethanoic acid and ethanol react with each others in the presence of conc. H₂SO4 to form ester and the reaction is called etherification.
■Ethanoic acid can neutralise NaOH. It can also decompose sodium carbonate and hydrocarbonate to liberate CO₂ gas
■Soaps are sodium salts of higher fatty acids containing 16-18 carbon atoms per molecule. They can be prepared by the Saponification reaction involving alkaline hydrolysis of oils and fats.
■Detergents are synthetic compounds which are ammonium salts or sulphonate salts or sulphates of long chain hydrocarbons containing 12-18 carbon atoms.
■Cleansing power of detergents is relatively more than that of soap.
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
23. What is meant by etherification reaction?
Ans. Etherification reaction is formation of ester molecule by the reaction between carboxylic acids and alcohols in the presence of sulphuric acid.
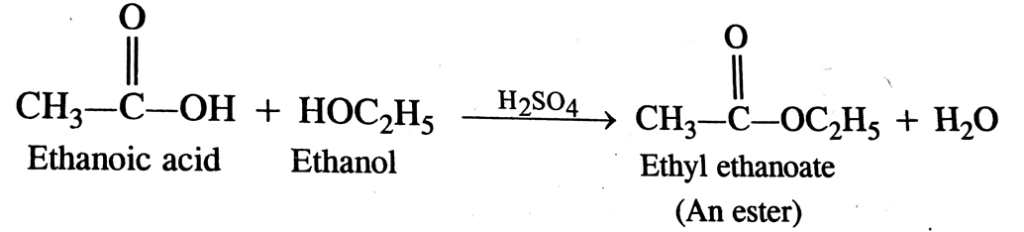
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
24. People use a variety of methods to wash clothes. Usually after adding soap, they beat the clothes on a stone on beat them with a paddle; scrub with brush or mixture is agitated in a washing machine. Why is this agitation necessary to get clean cloth?
Ans. When dirty clothes are soaked in soap solution, soap micelles containing the oily dirt at the centre are formed. In these micelles, soap is attracted both by the oily dirt and water. As a result, the surface tension of water decreases and a stable emulsion of oil in water is formed. To wash away the loosened dirt particles in form of micelles from the surface of the cloth, it is either scrubbed mechanically or beaten on a stone or with a paddle or agitated in a washing
24. Out of HCI and CH3COOH, which one is a weaker acid and why? Describe an activity to support your answer.
Ans. CH3COOH is a weaker acid than HCl. This is because of the reason. That HCl ionises in aqueous solutions to a larger extent whereas ionization of CH3COOH takes place to a smaller extent. Therefore, HCl gives more H+ ions per mole in water as compared to CH3COOH. Hence, HCl is a stronger acid.
Activity:
■Take HCl and CH3COOH in two different test tubes.
■Dip a strip of universal pH paper in each tube.
■In a tube containing HCl the colour of pH paper will become red where as in a tube containing CH3COOH, it will change to orange. This clearly shows that HCl is a stronger acid.
25. Name the oxidising agent used for the conversion of ethanol to Ethanoic acid.
Ans. The oxidizing agent used for converting ethanol to Ethanoic acid is aqueous solution of potassium dichromate containing sulphuric acid (K₂Cr₂O7/H₂SO4) or alkaline potassium permanganate (KMnO4) solution.
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
26. Write the chemical name and formula of vinegar.
Ans. Vinegar is a dilute aqueous solution (about 5-20%) of Ethanoic acid. The formula of acid is CH3COOH.
27. Write one advantage and disadvantage of using ethanol.
Ans. Advantage: Ethanol is the starting material for the manufacture of a large number of substances like chloroform, Ethanoic acid and ether.
Disadvantage: People generally consume ethanol in the form of alcoholic beverages. Excessive and regular consumption of ethanol is extremely injurious to human system. It can damage vital organs like liver and kidneys.
28. Explain the action of soap in removing an oily spot from a piece of cloth.
Or
Explain the mechanism of cleansing action of soap.
Or
State briefly how the formations of micelles help to clean the clothes having oily spots.
Ans. Dirt is generally held to the surface of the dirty cloth by a thin film of oil or grease. When a dirty cloth is dipped in soap (or detergent) solution. The non polar hydrocarbon chains of soap molecule dissolve in oil or grease while the polar heads of soap molecules get attracted by surrounding water. As a result soap micelles are formed with oily or greasy dirt lying at their centers. In other words, a stable emulsion of oil in water is formed.
When the surface of cloth is mechanically scrubbed or agitated the loosened oil dirt particles are removed from the dirty surface and the cloth is cleaned.
29. What are micelles? How is micelle formed when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Ans. Micelles: Micelle is a cluster or an aggregate formed in colloidal solution by the surface active molecules such as soap or detergent molecule in such a way that their polar heads remain on the surface of cluster while polar tails are directed towards the interior of cluster.
Formation of micelles when soap is added to water.
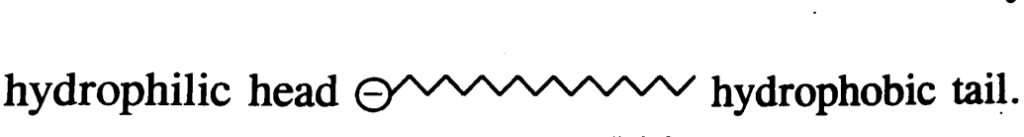
Soap and detergent molecules consist of two parts.
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
(i) Non polar hydrocarbon chain which is insoluble in water and is also called hydrophobic tail.
(ii) Negatively charged head which is water soluble and is called hydrophilic head as shown below.
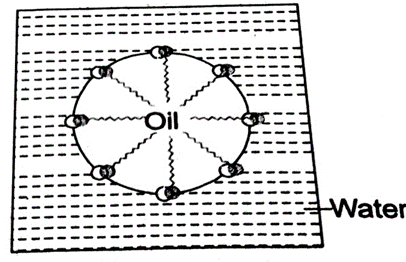
On adding soap to water, the polar hydrophilic heads of the soap molecules dissolve in water while non-polar hydrophobic chains attract one another and come close to form a cluster called micelle.
Micelle formation in ethanol: In solvents like ethanol, micelle formation will not occur because the non-polar chains will also dissolve in ethanol. Therefore cluster formation will not take place.
30. Why are detergents better cleansing agents than soaps?
Ans. Detergents lower the surface tension of water relatively to a larger extent as compared to soaps. Also detergents can safely be used in hard water where use of soap is difficult.
31. Intake of small quantity of methanol can be lethal. Comment.
Ans. Drinking of methanol is lethal because in the liver, it gets oxidised to methanol (formaldehyde) which rapidly reacts with the components of the cell. As a result, protoplasm gets coagulated in much the same way as an egg gets coagulated on boiling or cooking. Consequently, it leads to death.
32. Write the chemical equation for the reaction of Ethanoic acid with the following:
(a) Sodium
(b) Sodium hydroxide
(c) Ethanol
Ans. (a) 2CH3COOH (aq) + 2Na(s)
2CH3COONa (aq) + H2 (g)
Ethanoic acid Hydrogen
(b) CH3COOH (aq) + NaOH (aq) CH3COONa (aq) + H2O (l)
Sodium ethanoate
(c) CH3COOH + C2H5OH CH3COOC2H5 + H2O
Ethyl ethanoate
ALSO VISIT :
10TH CBSE
| Also Learn: | Attempt the Quiz on Mathematics | Attempt the Quiz on Social Science (Economics) | |
| GLOBALISATION AND INDIAN ECONOMY MONEY AND CREDIT MIND MAP FOR NATIONALISM IN EUROPE LIGHT REFLECTION AND REFRACTION REAL NUMBERS | Real Numbers | Development Sectors of Indian Economy Money and Credit Globalisation and Indian Economy Consumer Rights |
DOWNLOAD MOBILE APPLICATION TO LEARN MORE: QUESTIONS ON CARBON AND ITS COMPOUNDS
