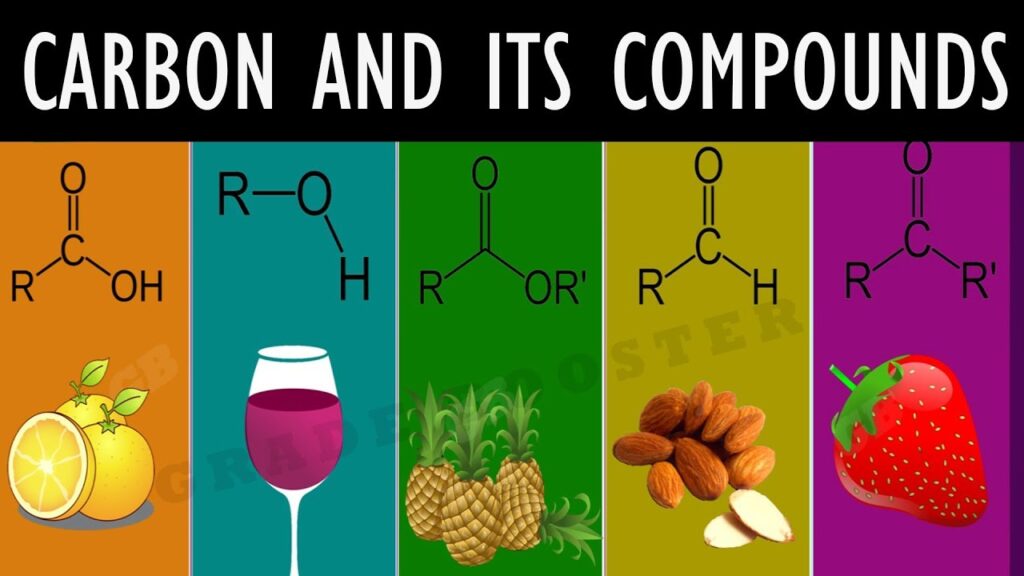
1.
Which of the following does not belong to the same homologous series?
2.
Carbon forms four covalent bonds by sharing its four valence electrons with four univalent to e.g., hydrogen. After the formation of four bonds, carbon attains the electronic configuration of
3.
Mineral acids are stronger acids than carboxylic acids because (i) mineral acids are completely ionised (ii) carboxylic acids are completely ionised (iii) mineral acids are partially ionised (iv) carboxylic acids are partially ionised
4.
Identify the unsaturated compounds from the following (i) Propane (ii) Propene (iii) Propyne (iv) Chloropropane
5.
The first member of alkyne homologous series is
7.
Carbon exists in the atmosphere in the form of
9.
While cooking if bottom of the vessel is getting blackened in the outside, it means that
11.
Chlorine reacts with saturated hydrocarbons at room temperature in the
12.
Butanone is a four carbon compound with functional
15.
Which of the following statements are usually correct for carbon compounds? These (i) Are good conductors of electricity (ii) Are poor conductors of electricity (iii) Have strong forces of attraction between their molecules (iv) Do not have strong forces of attraction between their molecules
16.
Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of
17.
Buckminster fullerene is an allotropic form of
18.
In which of the following compounds, -OH is the functional group?
19.
A molecule of ammonia (NH3) has
20.
Ethanol reacts with sodium and forms two products. These are