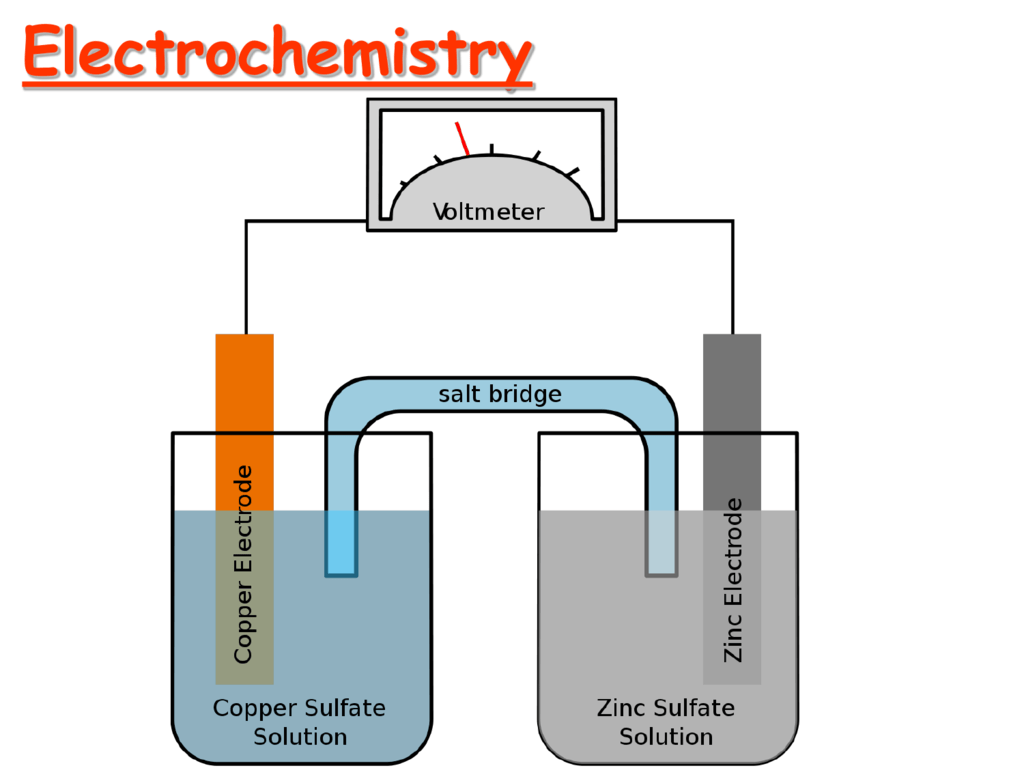
3.
A solution of potassium bromide is treated with each of the following. Which one would liberate bromine?
10.
Standard electrode potentials of three metals X, Y and Z are –1.2 V, + 0.5 V and – 3.0 V respectively. The reducing power of these metals will be
15.
Consider the following relations for emf of an electrochemical cell
(i) EMF of cell = (Oxidation potential of anode) – (Reduction potential of cathode)
(ii) EMF of cell = (Oxidation potential of anode) + (Reduction potential of cathode)
(iii) EMF of cell = (Reductional potential of anode) + (Reduction potential of cathode)
(iv) EMF of cell = (Oxidation potential of anode) – (Oxidation potential of cathode)
Which of the above relations are correct?