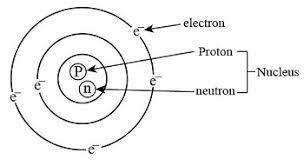
1.
How will fund the valency of magnesium?
2.
An element X has a mass number 4 and atomic number 2. Write the valency of the element.
3.
Is it possible for the atom of an element to have one electron, one proton and no neutron?
4.
Isotopes of an element have:
5.
How will find the valency of sulphur?
6.
Which of the following statement/s about Rutherford’s model of atom are correct? i. Considered the nucleus as positively charged ii. Established that the alpha particles are four times as heavy as a hydrogen atom. iii. Can be compared to solar system iv. Was in agreement with Thomson’s model.
7.
Which of the following are true for an element? i. Atomic number = number of protons + number of electrons ii. Mass number = number of protons + number of neutrons iii. Atomic mass = number of protons = number of neutrons iv. Atomic number = number of protons = number of electrons
8.
The electron distribution in an aluminium atom is:
10.
Which of the following correctly represents the electronic distribution in the Mg atom?
12.
Which of the following statements is always correct?
13.
Rutherford’s alpha particles scattering experiment resulted into discovery of:
14.
J.J Thomson proposed that the nucleus of an atom contain only nucleons.
15.
Elements with valency 1 are:
16.
Rutherford’s alpha-particle scattering experiment was responsible for the discovery of:
17.
Isotope of iodine is used for making tincture iodine, which is used as a medicine.
18.
The first model of an atom was given by:
19.
Atomic models have been improved over the years. Arrange the following atomic models in the order of their chronological order: i. Rutherford’s atomic model ii. Thomson’s atomic model iii. Bohr’s atomic model
20.
If Z = 3, what would be the valency of the element?
21.
The atomic number of calcium and argon are 20 and 18 respectively, but the mass number of both these elements is 40. What is the name given to such a pair of elements?
23.
The ion of an element has 3 positive charges, mass number of atom is 27 and the number of neutrons is 14. What is the number of electron in the ion?
24.
Rutherford’s alpha particle scattering experiment showed that: i. Electrons have negative charge. ii. The mass and positive charge of the atom is concentrated in the nucleus. iii. Neutron exists in the nucleus. iv. Most of the space in atom is empty v. Which of the above statements are correct?
25.
A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
26.
How will find the valency of chlorine?
27.
An atom with 3 protons and 4 neutrons will have a valency of:
28.
The mass of an electron is about 1 / 2000 times that of proton
30.
Dalton’s atomic theory successfully explained: i. Law of conservation of mass ii. Law of constant composition iii. Law of radioactivity iv. Law of multiple proportions